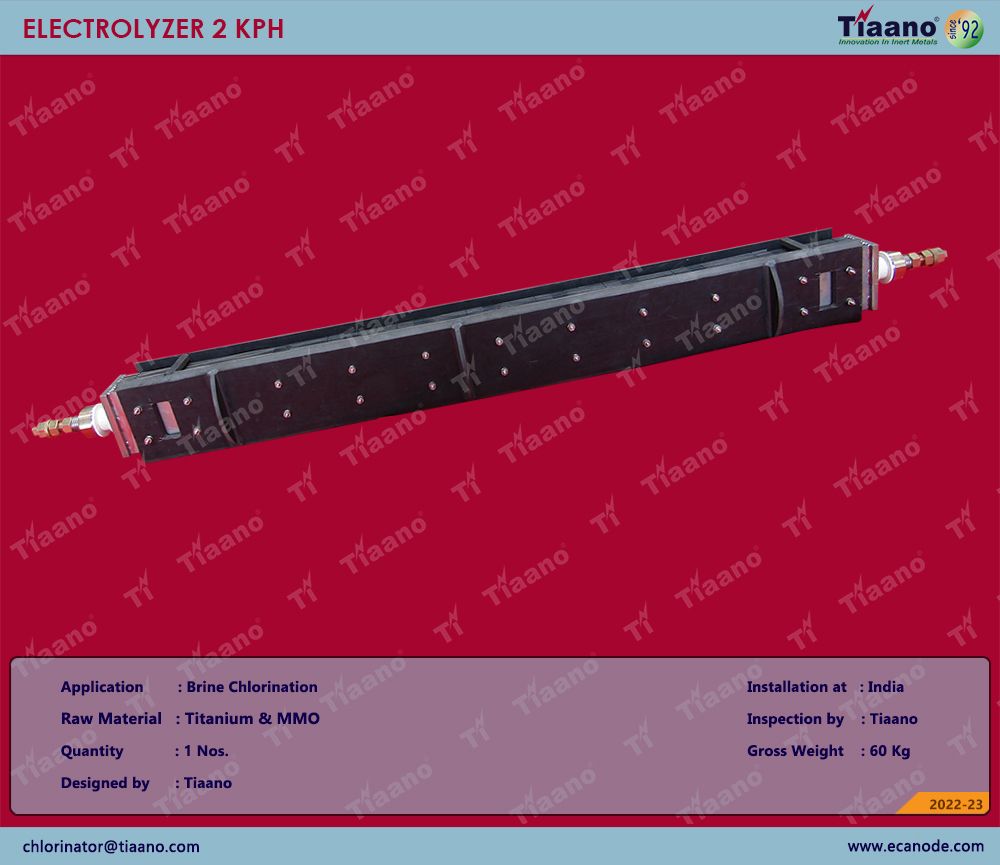
Manufacturing and Supply of Electrolyzer 2 KPH for Brine Chlorination
August 30, 2022 at 10:16 AM
—
admin
Electro chlorination is the process of producing sodium hypochlorite on site using salt water. Through electrolysis, the process of Electro chlorination is a direct current electrical supply which provides the energy to discharge the ions from the electrolyte (salt solution). The electrochemical reaction results in free chlorine being generated at the anode, while hydrogen and sodium hydroxides are released at the cathode. The chlorine and sodium hydroxide react with each other further to form sodium hypochlorite, a salt water disinfectant.
· Reduced operational cost.
· Consistent solution strength.
· Production of required volumes as needed.
Electrolysis may sound at first like a high school laboratory experiment with beakers, a few wires and a couple of batteries, and we would not be wrong. But the impact of this process, which allows molecules to be broken down using electricity, in this case water molecules, is key to obtaining green hydrogen.
What is Electrolyzer process?
Electrolysis is a promising option for carbon-free hydrogen production from renewable and nuclear resources. Electrolysis is the process of using electricity to split water into hydrogen and oxygen. This reaction takes place in a unit called an Electrolyzer.
An Electrolyzer is a system that uses electricity to break water into hydrogen and oxygen in a process called electrolysis.
Contact us: chlorinator@tiaano.com
Mobile no: 9382512010