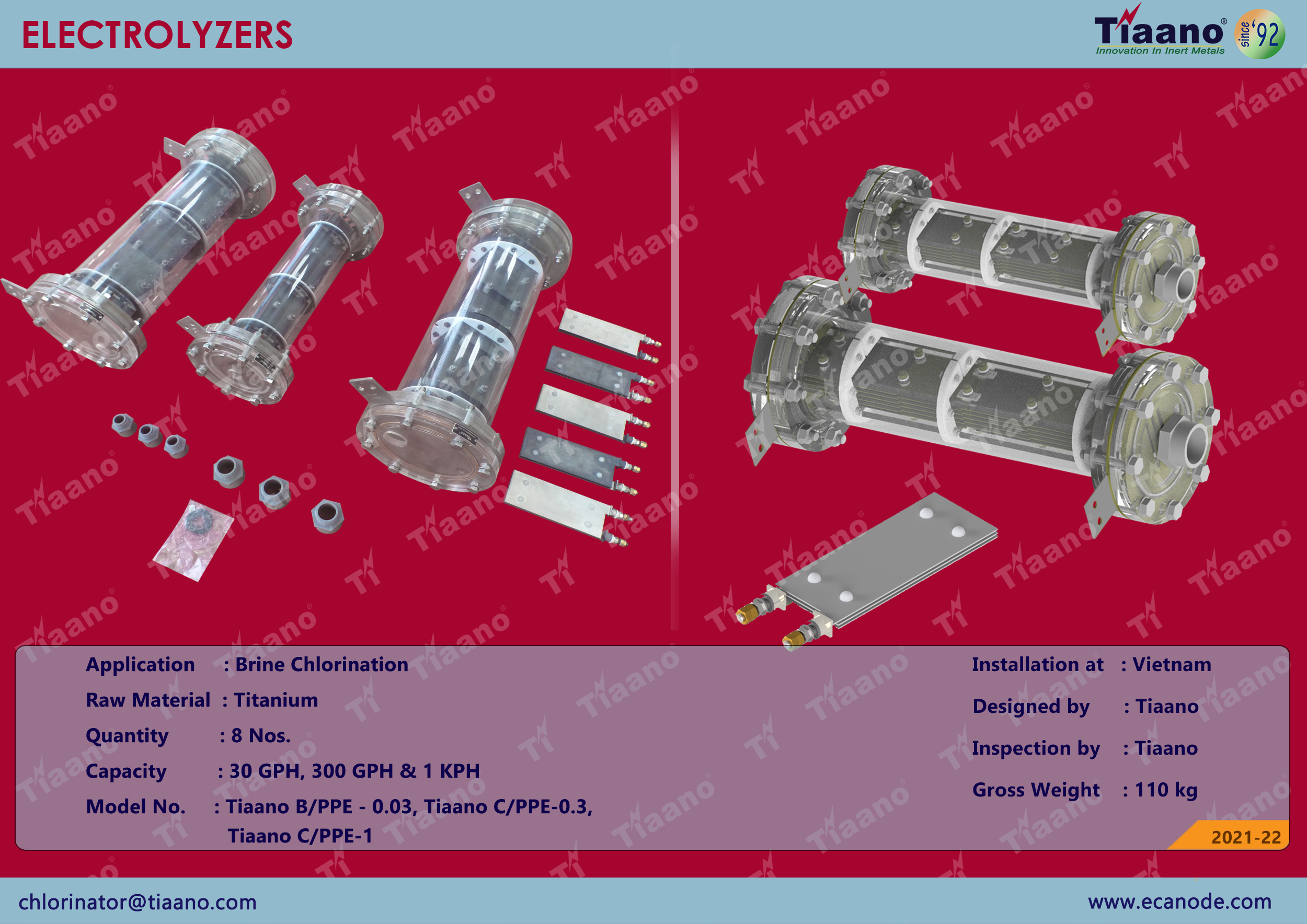
Electrolyzer 30 GPH, 300 GPH & 1 KPH
July 3, 2021 at 11:20 AM
—
admin
Electrochlorination is the process of producing sodium hypochlorite on site using salt water. Through electrolysis, the process of Electrochlorination is a direct current electrical supply which provides the energy to discharge the ions from the electrolyte (salt solution). The electrochemical reaction results in free chlorine being generated at the anode, while hydrogen and sodium hydroxides are released at the cathode. The chlorine and sodium hydroxide react with each other further to form sodium hypochlorite, a salt water disinfectant.
- Reduced operational cost.
- Consistent solution strength.
- Production of required volumes as needed.
A low voltage DC current is passed through the solution. At the positive anode, chloride ions are oxidized to produce chlorine. At the negative cathode, the salt water is reduced to sodium hydroxide and hydrogen. The liberated chlorine reacts instantly with the sodium hydroxide to produce sodium hypochlorite, while the hydrogen gas is released.
#user friendly operation and #safety #environment. Electro chlorination is user friendly. Gas chlorination and Tablets are tough to handle and you need experts to perform the same.
You can get more details on our Social Media page:-
https://www.facebook.com/Electrochlorinator
https://www.instagram.com/ec.anode/
https://www.pinterest.it/TiaanoElectrochlorinator/_saved/